U.S regulators have approved the first drug treatment for moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
The U.S Food and Drug Administration (FDA) on Friday, December 20 said that Zepbound (tirzepatide) will be used in combination with a reduced-calorie diet and increased physical activity.
“Today’s approval marks the first drug treatment option for certain patients with obstructive sleep apnea,” said U.S FDA official Sally Seymour. “This is a major step forward for patients with obstructive sleep apnea.”
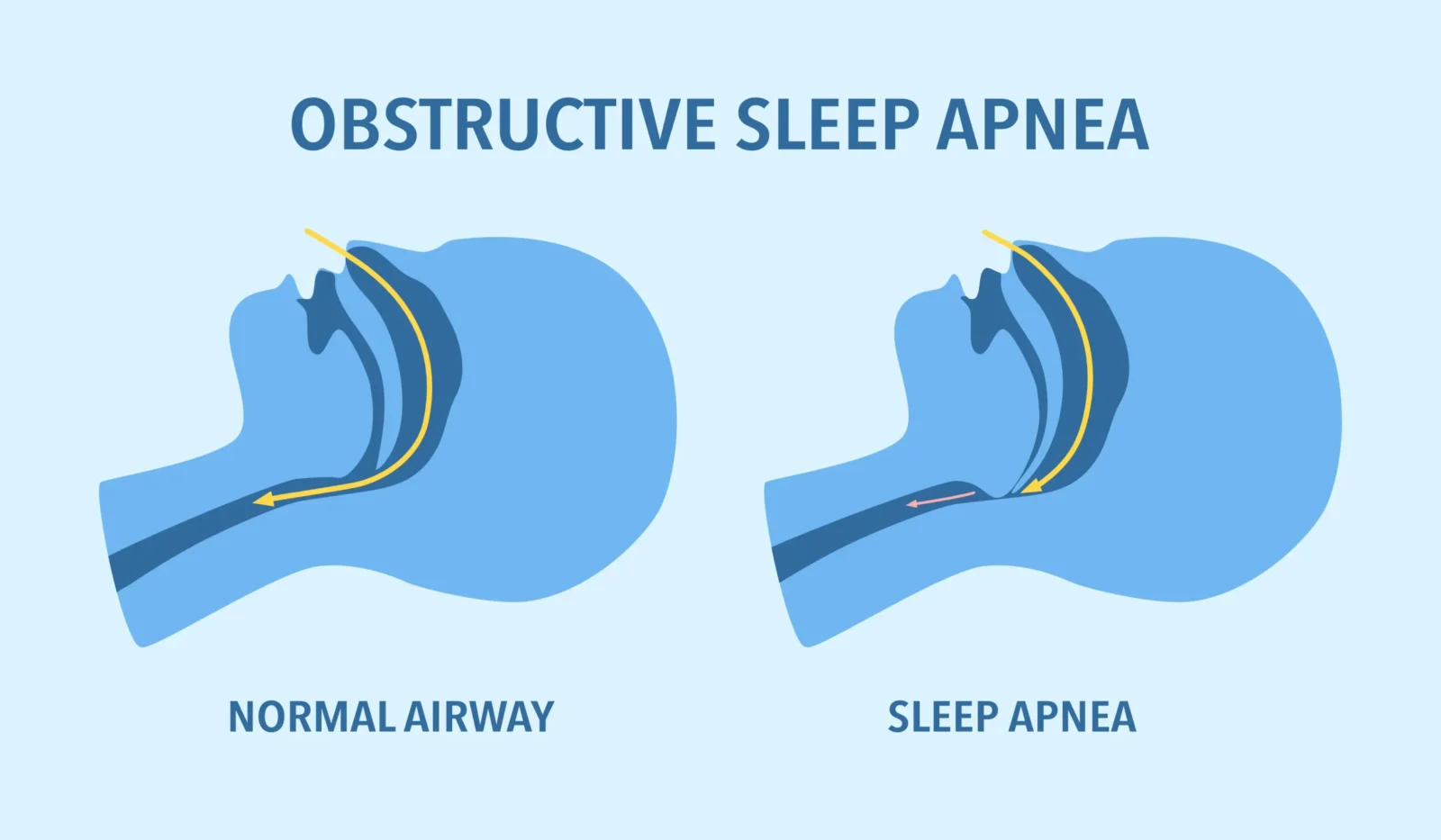
OSA occurs when a person’s upper airway becomes blocked, causing pauses in breathing during sleep. While OSA can affect anyone, it is more common in people who have overweight or obesity.
Zepbound, the U.S FDA says, works by activating receptors of hormones secreted from the intestine (glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP)) to reduce appetite and food intake. By reducing body weight, studies show that Zepbound also improves OSA.
Zepbound side effects
Zepbound can cause side effects such as nausea, diarrhea, vomiting, constipation, abdominal (stomach) discomfort and pain, injection site reactions, fatigue, hypersensitivity (allergic) reactions (typically fever and rash), burping, hair loss and gastroesophageal reflux disease.
Zepbound causes thyroid C-cell tumors in rats. It is unknown whether Zepbound causes such tumors, including medullary thyroid cancer, in humans. Zepbound should not be used in patients with a personal or family history of medullary thyroid cancer or in patients with Multiple Endocrine Neoplasia syndrome type 2.
Zepbound should not be used in patients with a history of severe allergic reaction to tirzepatide (its active ingredient) or to any of its other ingredients. Patients should stop Zepbound immediately and seek medical help if a severe allergic reaction is suspected.
“Zepbound also contains warnings for inflammation of the pancreas (pancreatitis), gallbladder problems, hypoglycemia (blood sugar that is too low), acute kidney injury, diabetic retinopathy (damage to the eye’s retina) in patients with type 2 diabetes mellitus, suicidal behavior or thinking, and pulmonary aspiration during general anesthesia or deep sedation. Patients should discuss with their health care provider if they have symptoms of pancreatitis or gallstones. If Zepbound is used with insulin or a medication that causes insulin secretion, patients should speak to their health care provider about potentially lowering the dose of these other medicines to reduce the risk of hypoglycemia. Health care providers should monitor patients with kidney disease, diabetic retinopathy and depression or suicidal behaviors or thoughts. Patients taking Zepbound should inform healthcare providers of any planned surgeries of procedures,” the U.S FDA says in a statement.